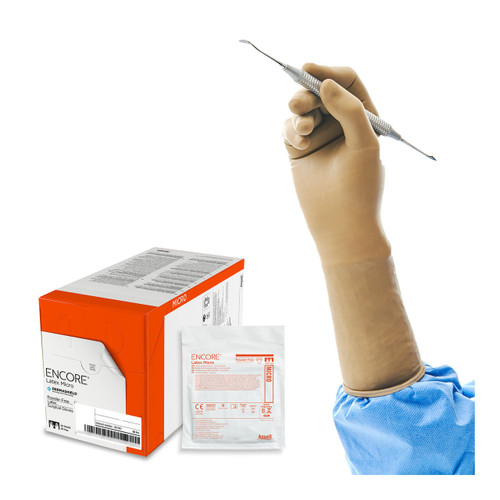
Product Overview
Pouch: 1 Pair (2 Gloves)
Pack: 25 Pouches (50 Gloves)
Case: 4 Packs (200 Gloves)
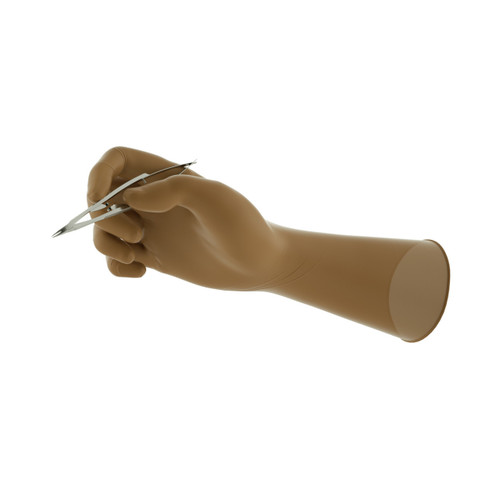
Ansell Encore® Microptic Sterile Natural Latex Surgical Gloves provide exceptional dexterity and tactile sensitivity.
Size: 7.5
Sterile
Contains Latex
Features & Benefits:
• Suitable for double gloving.
• Low protein content.
• DERMASHIELD™ Technology inner coating for fast and easy damp or dry donning.
• Powder free.
Suitable For:
• Delicated procedures such as in ophthalmology, micro, and cardiovascular
• Hopsital & Surgical Centers
• Speciality Healthcare
• Dental
• Animal Health
• First Responders
Specification:
Material: Natural Rubber Latex
Powder Content: Powder Free
Colour: Brown
Thickness: Micro
Shape: Anatomic with curved fingers
Cuff Style: Beaded with SUREFIT™ Technology
External Glove Surface: Smooth with micro-textured finish
Internal Glove Surface: Polymer Coated with DERMASHIELD™ Technology
Allergy Prevention: None
Grip Level: Moderate
Double Gloving Recommendation: Outer glove or underglove
Tested For Use With Chemotherapy Drugs: Yes, in accordance with ASTM D6978
Freedom from Holes (Inspection level I): 0.65 AQL
Sterilization Method: GAMMA irradiation (25 kGy)
Viral Penetration: Passes ASTM F1671 using Phi X 174, Passes ISO 16604 using Phi X 174
Product Standards: ASTM D3577, ASTM D7160, EN 455 Part 1-4, EN ISO 374-1, EN 374-2 and -4, EN 16523-1, EN ISO 374-5, EN 420, ISO 10282
Quality/Environmental Standards: ISO 9001, ISO 13485, ISO 14001, ISO 11137-Part 1, EN 556
Product Certification: EU: CE Marked to MDD 93/42/EEC (Class IIa), PPE Regulation (EU) 2016/425 (Cat. III risks) US: 510K # K000295, Class I/CA: MDALL # 14561, Class 2
Packaging: 50 pairs per box; 4 boxes per carton/case; 200 pairs per carton/case
Shelf Life: 3 years
Storage Instructions: Keep out of direct sunlight; store in a cool and dry place. Keep away from sources of ozone or ignition.
Disposal Recommendations: Gloves and pouch should be disposed of as clinical waste. Paper inner wrap, box and carton/case are recyclable but can be disposed of as clinical waste.
Vulcanization Chemical Accelerators: Zinc Dibutyldithiocarbamate (ZDBC), Zinc Diethyldithiocarbamate (ZDEC)
Primary Skin Irritation: Not considered a primary irritant as per FHSA Regulation guidelines 16 CFR 1500 / ISO 10993-10
Skin Sensitization: No evidence of delayed dermal contact sensitization as per ISO 10993-10
Protein Level: 30 ?g/g or less of total extractable protein